
- Industry news
Industry news
- Category news
- Reports
- Key trends
- Multimedia
Multimedia
- Journal
- Events
- Suppliers
Suppliers
- Home
- Industry news
Industry news
- Category news
- Reports
- Key trends
- Multimedia
Multimedia
- Events
- Suppliers
Suppliers
Kirin and Tokyo Uni discover cellular aging reduces nutrient absorption using biomimetic tech
Key takeaways
- Kirin and the University of Tokyo have found that cellular aging in intestinal epithelial cells reduces nutrient absorption, using biomimetic intestinal organoids.
- The study identifies EMT as a key mechanism linking intestinal aging to reduced uptake of glucose and amino acids.
- The findings could inform the development of functional ingredients targeting intestinal health to support nutrition, longevity, and healthy aging in older adults.

Kirin Holdings and the University of Tokyo, Japan, have discovered that nutrient absorption decreases in aging intestinal epithelial cells. They say this finding is a global first, which may potentially lead to functional ingredient innovations to subdue intestinal aging.
The researchers used a model of an artificial intestinal organoid derived from induced pluripotent stem cells (iPS) to identify epithelial-mesenchymal transition (EMT), a key mechanism of the aging process.
The researchers warn that reduced nutrient absorption can lead to systemic health issues such as frailty.
“The impact of aging on nutrient absorption may vary depending on the type of nutrient. Moving forward, it will be important not only to increase intake but also to determine the optimal composition and formulation for older adults,” Tatsuhiro Ayabe, Ph.D., Central Research Institute, R&D Division, at Kirin Holdings, tells Nutrition Insight.

Kirin aims to create new functional ingredients to support intestinal health. It believes that targeting intestinal health is a foundation for longevity, as it is linked to systemic aging, and reduced nutrient absorption contributes to malnutrition in the elderly.
“Nutritional function may change throughout life, so we believe that nutrition strategies should be optimized for each individual and life stage rather than relying solely on dietary intake,” adds Ayabe.
Research on aging
Kirin notes that research on aging is becoming more critical for super-aged societies. However, studying intestinal aging has been challenging because it is difficult to evaluate mechanisms in elderly people.
Decreased expression of genes related to nutrient absorption associated with cellular senescence.This is why research and understanding of intestinal aging has remained limited until now.
Kirin explains that EMT causes epithelial cells on the intestinal tract lining to lose epithelial characteristics and acquire traits of mesenchymal cells, which are more loosely organized than epithelial cells. This means that the tract lining may have a weaker barrier function against external threats.
Although EMT can help in wound healing, in excess amounts, it is linked to pathological conditions, including tissue fibrosis and the progression of cancer cell invasion and metastasis, warns Kirin.
The study found that lower gene expression, induced by aging cells, is associated with reduced nutrient uptake and increased EMT-related gene expression.
“This study suggests that EMT may be a more direct cause of declining nutritional function than cellular aging itself,” says Ayabe. “We believe this finding will be crucial for identifying appropriate targets as we advance the development of solutions focused on nutritional function.”
“Human nutrition remains an area where many molecular-level mechanisms are not fully understood. Utilizing biomimetic technologies, including intestinal organoids, offers the potential to enhance the reliability of evidence.”
However, challenges remain in translating cellular mechanisms into effective functional ingredients.
“Based on the mechanism identified in this study, a key challenge is determining whether the effects experienced by individuals who consume functional ingredients can be evaluated using measurable biological indicators,” adds Ayabe.
Study details
In the study, researchers triggered cellular senescence in human small intestinal organoids by cisplatin treatment, an anticancer drug. Quantitative PCR was used to assess gene expression and EMT. This enables DNA amplification while counting how much DNA researchers started with.
The team used gene expression linked to nutrient absorption to compare normal human intestines with senescent organoids.
Researchers found that cell aging caused a reduction in the gene expression of SLC5A1, which is involved in glucose absorption, and SLC16A10, which is involved in amino acid absorption.
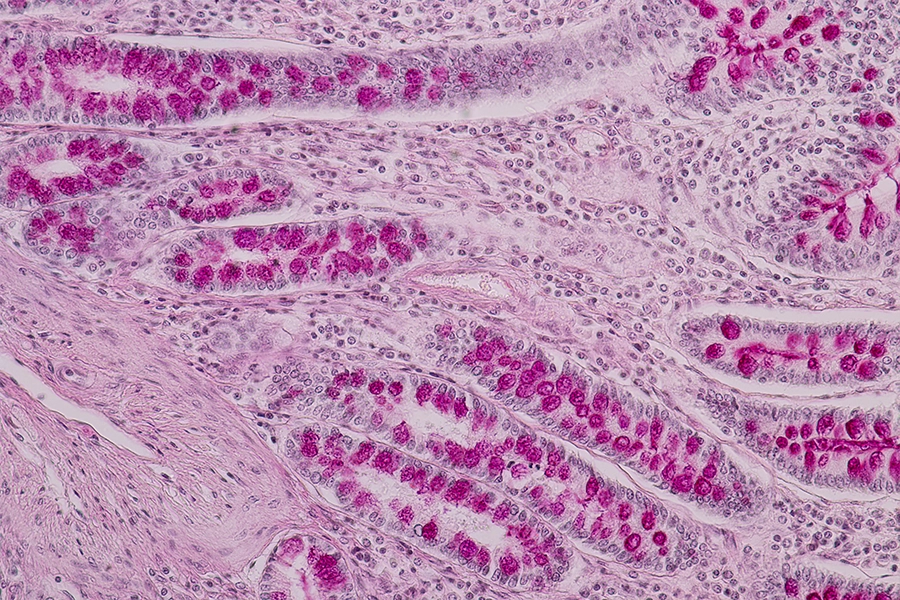 Biomimetic intestinal organoids to show that cellular aging reduces nutrient absorption, highlighting new targets for healthy aging solutions.Mechanisms affecting nutrient absorption were found to increase expression of TGFB1, an EMT-causing gene, and mesenchymal cell marker genes (ACTA2 and CDH2).
Biomimetic intestinal organoids to show that cellular aging reduces nutrient absorption, highlighting new targets for healthy aging solutions.Mechanisms affecting nutrient absorption were found to increase expression of TGFB1, an EMT-causing gene, and mesenchymal cell marker genes (ACTA2 and CDH2).
Kirin and the University of Tokyo presented their findings at the 48th Annual Meeting of the Molecular Biology Society of Japan last month.
Longevity in focus
As personalized nutrition and longevity goals gain momentum, genomics can help consumers fine-tune supplementation strategies based on their genetic makeup.
In recent healthy aging developments, PureHealth Research launched Youth Switch longevity, addressing a fundamental aging marker — telomere shortening. The supplement is a specialized blend of adaptogens and antioxidants that have been studied for their effects on telomerase activity and cellular regeneration.
Also, a clinical study found that Euromed’s Pomanox pomegranate extract improved a key biomarker of healthy aging. Meanwhile, a separate paper revealed caffeine might play a role in slowing down the aging process at a cellular level.
Meanwhile, dsm-firmenich told Nutrition Insight that research shows many consumers understand the role of diet and nutrition in healthy aging, with over half (52%) explicitly associating health expectancy with nutrition and wellness.














