From bacteria to seaweed: New gels support ethical, animal-free nutrition research
Key takeaways
- Researchers in the Netherlands and the US created synthetic and seaweed-based scaffolds to replace animal-derived materials like Matrigel.
- The synthetic PIC-Invasin gel supports long-term organoid growth and improves reproducibility for gut and metabolism research.
- Seaweed-derived scaffolds offer a sustainable, vegan, and biocompatible alternative for 3D cell cultures.

Researchers in the US and the Netherlands have developed synthetic and natural animal-free gels. The findings align with the search for more ethical and reproducible tools in nutrition and food science.
Nutrition Insight speaks to the researchers about how their materials support cell and organoid growth without using animal-derived materials.
Many nutrition and biomedical studies rely on animal-based materials such as Matrigel and collagen, which can vary between batches and affect reproducibility. These materials are often used to model nutrient absorption, gut barrier function, or microbiome interactions.
Researchers from Oregon State University, US, use seaweed to create a more sustainable and ethical material for human-relevant research models.
Meanwhile, Dutch researchers from the Hubrecht Institute and Radboud University combined bacterial protein Invasin with the synthetic gel polyisocyanopeptide (PIC) to create an environment for long-term organoid growth.
Synthetic gel replaces mouse-derived Matrigel
Published in Proceedings of the National Academy of Sciences, the study’s first author from the Netherlands, Joost Wijnakker, Ph.D. candidate at Hubrecht Institute, tells us that tumor cells grown in mice’s abdomen secrete Matrigel or BME.
“Each batch contains thousands of proteins related to the introduced tumor cells — and since every mouse is different, so is every batch.”
“In contrast, the PIC hydrogel developed by Professor Paul Kouwer (Radboud University) is fully synthetic and chemically defined — always identical in composition. The only added component is the single protein Invasin, which provides the necessary cell-binding functionality.”
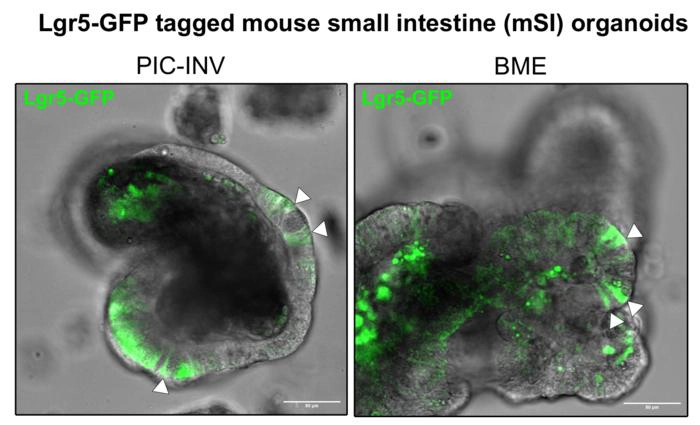 Mouse intestinal organoids grown in PIC–invasin and BME (Image credit: Wijnakker).Wijnakker explains that the PIC-invasin gel enables more consistent, reproducible, and completely animal-free organoid cultures, making them a more ethical alternative to traditional systems. He adds that it behaves identically to Matrigel.
Mouse intestinal organoids grown in PIC–invasin and BME (Image credit: Wijnakker).Wijnakker explains that the PIC-invasin gel enables more consistent, reproducible, and completely animal-free organoid cultures, making them a more ethical alternative to traditional systems. He adds that it behaves identically to Matrigel.
2D and 3D precise testing
The new animal-free gel could influence human gut organoid research, which is key to understanding nutrition and metabolism. Wijnakker points out that polarized intestinal epithelial cells are responsible for the gut’s metabolism and absorption of nutrients.
“Their apical side takes up and processes nutrients, but in traditional organoid systems — which form closed spheres — that side is often inaccessible. As the lumen, where nutrient uptake takes place, is enclosed by the spherical structure of the organoid.”
Wijnakker explains that last year’s 2D Invasin study showed that intestinal epithelial cells can grow directly on Invasin alone. “This creates an animal-free monolayer system that provides full access to both the apical and basal sides of the cells — ideal for studying nutrient uptake and gut barrier function.”
“The 2D Invasin system and the 3D Invasin-PIC hydrogel are complementary tools that together expand the possibilities for human gut organoid research.”
“The 2D Invasin culture format, with access to apical and basal sides, is particularly powerful for studying nutrient transport and microbiome interactions — both of which occur at the apical surface of the intestinal epithelium. This accessibility makes functional studies far more precise,” he adds.
Wijnakker states that epithelial and organoid cultures can be maintained long-term with Invasin-based systems, allowing multi-generational studies of nutrition and metabolism in vitro.
“Together, these advances mark a significant step toward fully animal-free epithelial and organoid culture, supporting ethical and reproducible biomedical and nutrition research.”
Vegan alternatives from seaweed
While the Dutch team developed a synthetic approach, US researchers turned to a natural marine material for an equally animal-free alternative. The study in Biointerphases shows how marine red seaweed, Pacific dulse, can be used after treatment with the reagent sodium dodecyl sulfate, commonly found in labs.
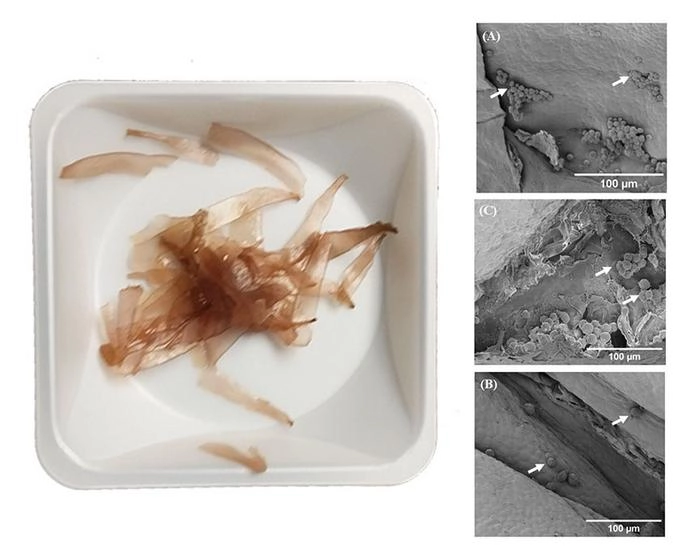 The seaweed sample before preparation and images of the seaweed scaffolds with new cells (Image credit: Chithiravelu).Study co-author Gobinath Chithiravelu, graduate research assistant at Oregon State University, US, tells us: “One interesting application is using seaweed-based scaffolds to grow cell-based proteins or to test nutrients in a 3D environment that mimics tissues.”
The seaweed sample before preparation and images of the seaweed scaffolds with new cells (Image credit: Chithiravelu).Study co-author Gobinath Chithiravelu, graduate research assistant at Oregon State University, US, tells us: “One interesting application is using seaweed-based scaffolds to grow cell-based proteins or to test nutrients in a 3D environment that mimics tissues.”
“This approach could give us more accurate models compared to the traditional 2D culture we’ve been using. It has the potential to advance cellular agriculture, especially in fields like cultivated meat and nutrient testing, where having a structured end product is crucial,” he highlights.
Chithiravelu underlines the significance of vegan alternatives to collagen and Matrigel, as animal-derived materials used in preclinical and nutrition research are ethically questionable, and expertise varies from batch to batch.
“Discovering a vegan alternative like seaweed or fungi could not only make research more ethical and cost-effective but also scalable, all while still providing a suitable environment for cell growth,” he comments.
“Instead of using Matrigel, we could use a mix of alginate and mycelium-derived hydrogel as a 3D scaffold for intestinal epithelial cells. This would allow us to study how plant-derived polyphenols and vitamins are absorbed without relying on animal products. It’s a game-changer for accurately modeling digestion and nutrient uptake in a sustainable way.”
“My focus is on developing reliable sources and materials that minimize our dependence on animal products, paving the way for quicker and more inclusive advancements in both medical and food research.”
Ethical, sustainable, and biocompatible alternatives
Seaweed is especially exciting for sustainability, says Chithiravelu, as it grows fast and doesn’t need arable land, freshwater, or fertilizers. “This makes it a low-impact biomaterial with a much smaller carbon footprint compared to animal-based collagens or gelatin, which heavily rely on livestock.”
“Plus, cultivating seaweed helps absorb carbon and nutrients in our oceans, highlighting its positive environmental impact. When it comes to biomaterial views, polysaccharides derived from seaweed, such as alginate, cellulose, carrageenan, and agarose, offer amazing benefits.”
“They are biocompatible, meaning they work well with living tissues, and they can be adjusted to create gels with specific properties like stiffness and porosity. This makes them perfect for applications like cell encapsulation and 3D cell cultures. Unlike animal-derived materials, they carry no risks of causing immune reactions or passing on diseases.”
He adds that synthetic materials are made from petroleum and require chemical alterations to make them safe for cells.
In conclusion, Chithiravelu says: “[Vegan scaffolds] may enable researchers to explore how nutrients are absorbed, how metabolism works, and how cells respond in a more precise and reliable manner.”
“I hope this encourages a shift toward in vitro human-based testing platforms, which would lessen our dependence on animal studies, at the same time enhancing the physiological relevance of nutritional research. It represents a significant step toward developing models that truly mirror human biology, with ethics and scientific soundness.”
Nutrition Insight previously spoke with the Physicians Committee for Responsible Medicine, which criticized US Institutional Animal Care and Use Committees for doing little to protect animals in research. PETA’s scientists flagged ethical biases and outdated assumptions in human nutrition research on the use of animals. We also spoke with experts, who touted the benefits of organ-on-chip technology.











